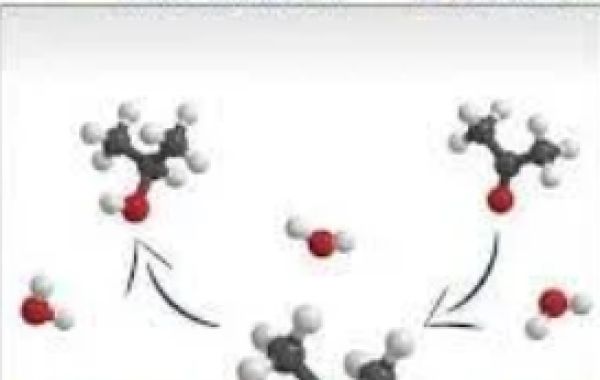
Chemical reactions are the driving force behind countless industrial processes, ranging from fuel production to drug discovery. However, many of these reactions require a catalyst to proceed at a satisfactory rate. In recent years, scientists have been exploring new types of catalysts that can enhance reaction efficiency while minimizing environmental impact. One promising innovation in this field is spherical methane catalysts - tiny particles with big potential. In this post, we'll explore how these innovative materials work and why they're increasingly being used in modern chemistry labs around the world!
What are spherical methane catalysts?
Spherical methane catalysts are a type of material that is used to speed up chemical reactions without being consumed in the process. These tiny particles, typically only a few nanometers in diameter, are made from materials such as metal oxides and sulfates.Unlike traditional catalysts, which often take the form of powders or larger chunks of material, spherical methane catalysts offer several advantages. First and foremost, their small size allows for greater surface area-to-volume ratios - meaning that more active sites are available for catalytic activity.Another key benefit of spherical methane catalysts is their ability to be easily dispersed in solution. This makes them ideal for use in applications such as liquid-phase hydrogenation - where they can be added directly into the reaction mixture to improve efficiency.While still relatively new on the scene compared to other types of catalysts, spherical methane catalysts show great promise when it comes to enhancing chemical reactions across a range of industries and laboratory settings.

How do they work?
Spherical methane catalysts work by providing a surface for chemical reactions to occur on. These catalysts are made up of tiny spheres that contain metal particles, such as platinum or palladium, which act as the active sites for the chemical reaction.When reactants come into contact with the surface of the spherical methane catalysts, they bind to the metal particles and undergo a series of chemical reactions. The energy required to break apart bonds in these molecules is lowered due to the presence of these metals, making it easier for new bonds to form.The shape and composition of spherical methane catalysts also play an important role in their function. Their small size means that they have a large surface area relative to their volume, allowing more reactant molecules to come into contact with them at once. Additionally, some metals are better suited than others for specific types of reactions.Spherical methane catalysts increase reaction efficiency by providing an optimized environment for chemical reactions to occur in. They allow reactions that would not otherwise be possible under normal conditions and can result in higher yields and selectivities while minimizing waste products.

The benefits of using spherical methane catalysts
Spherical methane catalysts have numerous benefits when it comes to enhancing the efficiency of chemical reactions. First and foremost, they are highly stable under reaction conditions due to their unique structural properties. This stability allows them to maintain their catalytic activity for long periods without degradation or deactivation.Moreover, spherical methane catalysts exhibit high selectivity in many reactions. This means that they can selectively promote certain reactions while suppressing others, leading to higher yields and reduced waste products. Their high selectivity also makes them ideal for use in complex multi-step syntheses where multiple products could be formed.Another advantage of using spherical methane catalysts is their ability to operate at lower temperatures compared to other types of catalysts. This not only saves energy but also reduces unwanted side-reactions that may occur at higher temperatures.These catalysts offer a cost-effective solution because they can be easily prepared from inexpensive starting materials such as methanol or formaldehyde.The use of spherical methane catalysts offers several benefits that make them attractive options for enhancing the efficiency of chemical reactions in various industries such as pharmaceuticals, petrochemicals, and fine chemicals manufacturing.
Some applications of spherical methane catalysts
Spherical methane catalysts have a wide range of applications in various industries. One of the most common applications is in the production of synthetic fuels, such as gasoline and diesel. Spherical methane catalysts help to improve the efficiency of these chemical reactions by increasing their speed and reducing energy consumption.Another important application of spherical methane catalysts is in the manufacture of plastics. These catalysts can be used to produce high-quality polymers with greater precision and control over their properties, leading to improved product quality and reduced waste.The pharmaceutical industry also benefits from using spherical methane catalysts. They are commonly employed in drug synthesis processes where they act as powerful agents for selective oxidation or hydrogenation reactions that make specific drugs more effective.Furthermore, spherical methane catalysts play an essential role in environmental protection through waste management procedures like water treatment plants, air purification systems, etc. They are instrumental in removing pollutants from industrial wastewaters while ensuring minimal harmful impacts on aquatic life forms.Spherical methane catalysts have numerous practical applications across various industries beyond what we've mentioned here today. With further technological advancements and research initiatives aimed at improving these catalytic materials' performance capabilities - it's only a matter of time before we see new breakthrough applications emerge!

Conclusion
Spherical methane catalysts have proven to be a revolutionary innovation in the world of chemical reactions. Their unique structure and properties make them highly efficient in accelerating reactions while reducing energy consumption and waste production.With numerous benefits such as high selectivity, stability, and low cost, spherical methane catalysts have found applications in various industrial sectors ranging from petrochemicals to pharmaceuticals. They are used to produce essential products like plastics, fuels, medicines as well as reduce harmful emissions into the environment.As technology advances, researchers continue to explore new ways of improving the performance of these catalysts through modifications on their surface area or crystal structure. It is exciting to imagine what future developments will come out from this field that could transform industries and enhance sustainability practices globally.It is clear that spherical methane catalysts hold immense potential for enhancing the efficiency of chemical reactions with minimal harm on our environment.If you want to know more about this product, please contact.camilleyxwn@outlook.com