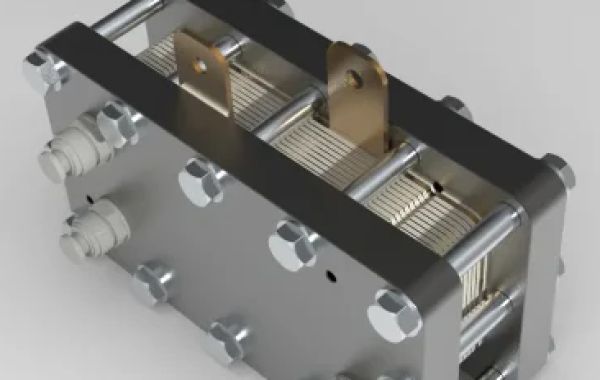
Unlocking the vast potential of hydrogen as a sustainable energy source is essential for our future. And PEM (Proton Exchange Membrane) electrolyzers have emerged as the top contender in producing this versatile fuel. Their advanced technology and advantages make them revolutionary in the field of hydrogen production. This post intends to shed light on what sets PEM electrolyzers apart, how they do their job, and why they are seen as the future of hydrogen production. So get ready to join Gatechn New Energy Technology (Shanghai) Co., Ltd. on this stimulating exploration of the amazing perks of using PEM electrolyzers!
How does a PEM electrolyzer work?
PEM electrolyzers, short for Proton Exchange Membrane Electrolyzers, are cutting-edge technologies that are essential for producing hydrogen gas. Water molecules are split into their constituent elements - hydrogen and oxygen - by undergoing an electrochemical process referred to as water electrolysis.
Essentially, a PEM electrolyzer consists of three components: an anode (positive electrode), a cathode (negative electrode), and a proton exchange membrane sandwiched between them. By using this unique setup, protons can be selectively transported while electrons are blocked.
During operation, an electric current is applied to the electrodes, triggering several simultaneous reactions. Oxidation occurs at the anode, where water molecules lose electrons to form hydrogen ions (protons) and oxygen gas. As these protons are combined with electrons from the external circuit at the cathode, pure hydrogen gas is formed simultaneously.
Compared to alkaline or solid oxide electrolyzers, PEM electrolyzers can operate at lower temperatures and pressures. By reducing the risks associated with high-pressure systems, this not only increases efficiency, but also enhances safety.
As a result of their ability to switch on and off quickly, PEM electrolyzers provide rapid response times. In applications where power demands are variable or intermittent energy sources, such as renewable energy integration, this flexibility enables efficient load following capabilities.
In addition to their technical advantages, PEM electrolyzers also excel in terms of scalability and versatility. They can easily be installed even in confined spaces, making them ideal for a variety of settings, including industrial plants and residential buildings seeking self-sufficiency via hydrogen generation on-site.
In the field of hydrogen production, PEM electrolyzers stand out as game-changers due to all of these remarkable characteristics. They are rapidly gaining traction as the preferred choice for harnessing hydrogen's potential due to their high efficiency levels, fast response times, enhanced safety measures, and adaptability across industries.

What is the working principle of a PEM electrolyzer?
Here's a look at how a PEM electrolyzer works and how it's revolutionizing hydrogen production.
Basically, a PEM electrolyzer works by electrochemically splitting water between two electrodes, an anode and a cathode, separated by a proton exchange membrane (PEM).
Water molecules are split into their constituent elements when electricity is applied to the electrolyzer: hydrogen gas at the cathode and oxygen gas at the anode. PEM plays a key role in this process.
During the reaction, the PEM serves both as a separator and a catalyst. Protons (H+) from one side pass through while electrons (e-) and other particles are blocked. As protons migrate across the membrane towards the cathode, they combine with electrons generated on that side to form hydrogen gas.
Unlike traditional alkaline or solid oxide electrolyzers, PEM electrolyzers can operate at lower temperatures and pressures, making them more efficient and safer.
Due to their ability to quickly switch on and off, they have faster startup times than other systems.
Since PEM electrolyzers can be easily adjusted according to varying hydrogen production demands, they offer greater flexibility in terms of scalability.
A PEM electrolyzer's ability to efficiently convert water into hydrogen gas using a proton exchange membrane opens up new possibilities for the generation of clean energy. In today's quest for sustainable energy solutions, PEM electrolyzers are gaining popularity because of their numerous benefits, including safety, efficiency, fast start-up times, and scalability.
Hydrogen production with a PEM electrolyzer
Its high efficiency is one of the most significant advantages of using a Proton Exchange Membrane (PEM) electrolyzer for hydrogen production. In order to ensure that the process is cost-effective and sustainable, PEM electrolyzers have an impressive conversion rate, ensuring they can efficiently convert electricity into hydrogen gas.
Another advantage of PEM electrolyzers is their compact size and portability. Unlike other types of electrolyzers, which tend to be bulky and require larger spaces, PEM electrolyzers are designed to be compact and lightweight. As a result, they are ideal for mobile hydrogen fueling stations as well as industrial hydrogen production on-site.
In addition, PEM electrolyzers offer fast response times when compared to other technologies. With their ability to switch between operational modes quickly, they can respond to fluctuating energy demands quickly, ensuring a stable supply of hydrogen when needed without compromising efficiency.
PEM technology also allows for a higher level of purity of hydrogen gas produced due to its selective permeability. The proton exchange membrane only allows protons and cations to pass through while blocking impurities such as gases or metal ions present in water sources used during the electrochemical reaction.
With no need for caustic chemicals or extreme conditions like high temperatures or pressures, PEM electrolyzers are considered safer than alternative methods.

Hydrogen production with PEM electrolyzers is the future
It has become increasingly important to find efficient methods of producing hydrogen as we seek to reduce carbon emissions and combat climate change. Hydrogen has emerged as a clean and sustainable energy source. Electrolyzers that use proton exchange membranes (PEMs) can help here.
The future of hydrogen production lies with PEM electrolyzers, which operate at lower temperatures and pressures than traditional alkaline electrolysis systems. They offer numerous advantages over other hydrogen production technologies. As a result, energy consumption is reduced and safety is improved during operation.
The precise control offered by PEM electrolyzers ensures optimal efficiency and cost-effectiveness as these devices respond quickly to fluctuations in demand.
Furthermore, PEM electrolyzers allow direct coupling with renewable energy sources, such as solar and wind power. By utilizing excess solar or wind energy during off-peak hours, PEM electrolyzers can store or inject green hydrogen directly into the grid.
Compact size and modular design of PEM electrolysers make them suitable for both centralized and distributed deployment scenarios, enabling flexibility in adapting to different infrastructure requirements.
Furthermore, PEM electrolysers produce high-purity hydrogen without any impurities or contaminants commonly found in other processes like steam methane reforming or coal gasification. As a result, hydrogen can be used in a number of applications, including fuel cells for transportation or industrial use.
It is evident that PEM electrolysers will play a crucial role in driving the transition towards a cleaner energy future, given advancements in materials science and manufacturing techniques.
Research and development efforts focused on advancing proton exchange membrane technology will accelerate its adoption across industries worldwide. By harnessing renewable resources more efficiently with advanced electrolysis technologies, such as Gatechn New Energy Technology (Shanghai) Co.,Ltd.'s PEM electrolysers
What you need to know about using a PEM electrolyzer
The use of a PEM electrolyzer for hydrogen production can provide numerous benefits to your energy needs. Here are some steps to get started with this highly innovative technology.
Understanding how a PEM electrolyzer works and its components, such as the proton exchange membrane and electrocatalysts, will help you make informed decisions throughout the process.
Determine the amount of hydrogen you need, as well as any other specific factors like purity or pressure levels. By knowing these details, you can select the appropriate size and capabilities of a PEM electrolyzer.
Identify your requirements and research reputable manufacturers or suppliers of high-quality PEM electrolyzers. Look for companies such as Gatechn New Energy Technology (Shanghai) Co.,Ltd. that offer advanced energy solutions.
Provide advice on system design, installation, and operation procedures tailored to your specific application needs. Experts can also assist in optimizing performance and ensuring safety protocols are followed.
Collaborate with partners or organizations who have experience integrating renewable energy sources into hydrogen production processes. By collaborating, you could leverage existing infrastructure or take advantage of funding opportunities in the clean energy industry.
Following the recommendations provided by experts or manufacturers, purchase and install your chosen PEM electrolyzer system once all preparations have been completed. Ensure optimal performance and longevity of your equipment by performing regular maintenance as prescribed by professionals.
As a result of these steps towards utilizing a PEM electrolyzer for hydrogen production effectively, you will not only contribute positively towards sustainable energy practices, but you will also decrease carbon emissions and increase overall efficiency.

Conclusion
Its advanced technology and numerous benefits make the Proton Exchange Membrane (PEM) electrolyzer a game-changer in the field of clean energy.
PEM electrolyzers produce hydrogen gas efficiently and with high purity by separating hydrogen ions from oxygen molecules using a solid polymer membrane. In addition to producing hydrogen with minimal impurities, this process works at lower temperatures and pressures than traditional methods.
Hydrogen production with a PEM electrolyzer has the benefit of being flexible. It can easily be scaled up or down according to demand, making it suitable for a variety of applications, from small-scale residential to large-scale industrial. Additionally, the rapid response time of PEM electrolyzers allows for quick adjustments based on fluctuating power supplies or market conditions.
In addition, the solid polymer membrane used in PEM electrolyzers minimizes the risk of gas leakage and explosion compared to other technologies. As a result of its compact design and lack of corrosive chemicals, maintenance requirements are significantly reduced over alternatives.
Furthermore, PEM electrolyzers promote sustainability and reduce carbon emissions. In order to produce green hydrogen without relying on fossil fuels, they use renewable energy sources during operation, such as solar or wind power. Consequently, they play an essential role in achieving a cleaner, more sustainable energy future.
Gatechn New Energy Technology (Shanghai) Co., Ltd., one of the leading manufacturers in this field, offers state-of-the-art PEM electrolyzers that combine efficiency, reliability, and environmental consciousness. Through their commitment to innovation, customers can actively contribute to the fight against climate change while embracing cutting-edge technology.